Animation & Explanation by BIGS With a click on the image above, the animation is ready to play. The animation is also available as an app at: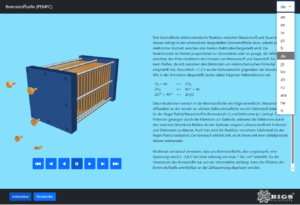
If you need immediate knowledge about „Fuel cell (PMFC)“,
please click on ChatGPT or DeepSeek.
Or you can check out this book recommendation on Amazon: Fuel cell
General Information on Fuel Cells
A controlled electrochemical reaction between hydrogen and oxygen to form water takes place in the schematically illustrated fuel cell as soon as an electrical connection is established between the two electrodes. The reaction rate is proportional to the current, meaning the resistance between the poles determines the consumption of hydrogen and oxygen.
A current can flow because an electrochemical potential is established between the electrodes, theoretically +1.2 V on the cathode side relative to the anode side. As shown in the animation, the following half-reactions occur:
O2 + 4e- => 2O2-
2H2 => 4H+ + 4e–
2O2- + 4H+ => 2H2O
These reactions occur in the fuel cell as follows:
Hydrogen gas diffuses to the anode’s active electrode layer, where a noble metal catalyst (typically platinum) splits the hydrogen into protons (H⁺) and electrons (e⁻). The protons pass through the membrane to the cathode, while the electrons flow through the external circuit.
At the cathode, oxygen from the air reacts with the protons and electrons to form water. This reaction is also catalyzed by a noble metal, usually platinum. The cycle is completed, generating electricity with water as the only byproduct.
It should be noted that each individual fuel cell, even when not stacked, produces a voltage of 0.3 – 0.8 V with a maximum power of 1 W/cm². Since the fuel conversion rate is determined solely by the current, the power output of the fuel cell can be directly read from the cell voltage.
Further knowledge on the topic of the quantum physics can be found in the book selection on Amazon:
Book Recommendation on Amazon: Fuel cell
Or, if you just want to browse a bit more on AliExpress or Amazon, click the following button:
To browse on AliExpress: Laptop discounted by 50%
To browse on AliExpress: Watch discounted by 87%
To browse on Amazon: NIKE shoes
In der Animation kann der Nutzer zwischen den folgenden Sorachen wechseln:
Deutsch, English (English), Spanisch (Español), Chinesisch (中国人), Russisch (русский), Arabisch, Koreanisch (한국어), Japanisch (日本), Französich ( français ), Türkisch ( Türk ) Indisch ( भारतीय ), Hebräisch ( עִברִית ) und Portugiesisch ( português ):
- Brennstoffzelle (PMFC);
- Fuel Cell (PMFC);
- La célula de combustible de membrana (PEMFC);
- 通过播放控制 (PEMFC); Топливный элемент (PMFC);
- خلية وقود الاحترا (PMFC);
- 연료전지 (PMFC);
- 燃料電池 (PMFC);
- Pile à combustible (PMFC);
- Yakıt hücresi (PMFC);
- ईंधन सेल: (PMFC);
- Célula de combustível (PMFC)
- This animation Fuel Cell exist in Hebrew and Arabic too.
